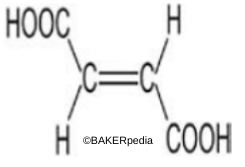
Chemical structure
Origin
Natural sources of fumaric acid include bolete mushrooms, lichen, and Iceland moss. It’s also found in papayas, pears and plums, but in very limited amounts. It was first isolated from the plant Fumaria officinalis, which is where it derives its name.1 Today, this acid is produced via chemical or enzymatic treatment of starchy materials.2
Its use as an acidulant dates back to 1946.
Function
In foods and baked goods, it serves many functions:
- Acidity control (measured by pH or more accurately by TTA)
- Highly effective acidulant
- Substitute for other acids such as citric, lactic, and tartaric1,2
- Strong buffering capacity around pH 3.04
- Antimicrobial / preservative activity
- Chemical leavening – highly effective, with a Neutralization Value of 145
- Dough machinability improver
- Flavor/ taste sourness enhancer
- Foam promoter in egg-white foams and a stabilizer of over-beaten egg whites
Commercial production
Fumaric acid is primarily produced using isomerization or biochemical routes.1
- Isomerization: this method is based on the conversion of maleic anhydride to maleic acid and the cis–trans isomerization of maleic acid to the final product.
- Biochemical: relies on bioconversion of substrates such as corn starch, corn straw, cassava bagasse or potato flour using maleate isomerase produced by Pseudomonas and Arthrobacter species.
Application
If you’re adding fumaric acid bakery products, here are a few things to keep in mind:
In bread, this acid is used mainly as an effective preservative and mold inhibitor. In artisan and sourdough breads, it imparts pleasant sour notes. It can also help improve dough rheology and enhance the formation of a porous crumb.
Mold growth is a major quality issue with tortillas. The addition of this ingredient is an essential mold growth inhibitor and shelf life extender, a result of its unique solubility profile. It can also act as a leavening acid. It is also used as an effective preservative in refrigerated biscuits and other baked goods.
It is added to English muffins to improve dough machinability and baked muffin porosity. In pie fillings, it d helps promote a smooth and optimal gelation due to extended critical cook time. In stove-top pudding mixes, fumaric acid is used as a coagulant.
One drawback of fumaric acid is its low solubility which can be improved by dissolving in hot water.
Regulations
The FDA considers fumaric acid and its calcium, ferrous, magnesium, potassium and sodium salts as GRAS food additives (21CFR172.350).3
In the EU and UK, fumaric acid is allowed. It has the E number E297.4
References
- Llica, R-A., Kloetzer, L. and Galaction,A-I. Fumaric acid: production and separation. Biochemistry Letters, 4 (2018): 18.
- Das, R.K., Brar, S.K., and Verma, M. Fumaric acid: production and application aspects. In: Brar SK, Sarma SJ, Pakshirajan K (eds) Platform chemical biorefinery: future green chemistry, edn. Elsevier, Amsterdam, (2016), pp 133–152.
- CFR – Code of Federal Regulations 21CFR172.350” Accessdata.fda.gov. 01 Apr.2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.350&SearchTerm=fumaric%20acid. Accessed 8 Nov 2019.
- COMMISSION REGULATION (EU) No 1129/2011 of 11 November 2011” eur-lex.europa.eu,https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32011R1129 . Accessed 21 Aug 2020.

