Chemical structure
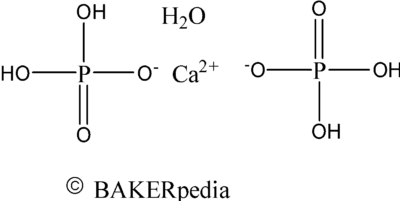
Function
MCP is a leavening acid with a neutralizing value of 80. It reacts with sodium bicarbonate and releases carbon dioxide in the presence of water. It is the preferred leavening acid because it doesn’t contain sodium and has no aftertaste.1
MCP is a quick-reacting leavening acid.1 It releases 60–70% of its carbon dioxide within the first few minutes of mixing. It is sometimes combined with slow-acting leavening acids, such as sodium aluminum sulfate, sodium acid pyrophosphate and sodium aluminum phosphate, in double-acting baking powders.1
It is used in products like pancakes, cookies and angel food cake mixes, where fast gas production and little bench time is desired. It is also used in biscuits and muffins when fast-acting leavening is needed due to short bake time.
MCP is a double-acting leavening acid. After two-thirds of the carbon dioxide is released during mixing, MCP is transformed to dicalcium phosphate, which is latent at room temperature but releases carbon dioxide when heat is experienced in the oven. Some brands of baking powder have MCP as the sole leavening acid.
Application
MCP should be used in conjunction with baking soda. The neutralizing value of leavening acids is the ratio of sodium bicarbonate (baking soda) to 100 parts of acid leavener that will bring about complete carbon dioxide release or “neutralization.”
For an acid with a neutralizing value of 80, if complete neutralization is desired, you would start out with a ratio of 80:100 parts baking soda : leavening acid. Adjusting the amount of leavening acid to baking soda can raise pH (decrease the acid amount) or lower pH (increase the acid amount) of the finished product. It is used in phosphate flour alone, and in self-rising flour with sodium bicarbonate.
FDA regulations
MCP is GRAS-regulated by the FDA in article 21CFR182.8217 in the Code of Federal Regulations.2 When monocalcium phosphate is used in phosphated flour, the amount allowed is between 0.25–0.75% of the weight of the finished phosphated flour.3 When it is used in self-rising flour, the total amount of sodium bicarbonate and MCP should not be more than 4.5% based on flour weight.4
References
- Chung, F.H. ”Leavening Acid Composition Produced by Heating Monocalcium Phosphate at Elevated Temperatures.” US Patent 5667836 A. 16 sept 1997.
- U.S. Food & Drug Administration. “21CFR182.8217 – Code of Federal Regulations Title 21.” Accessdata.fda.gov, 1 April 2017, www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.8217.
- U.S. Food & Drug Administration. “21CFR137.175 – Code of Federal Regulations Title 21.” Accessdata.fda.gov, 1 April 2017, www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=137.175.
- U.S. Food & Drug Administration. “21CFR137.180 – Code of Federal Regulations Title 21.” Accessdata.fda.gov, 1 April 2017, www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=137.180.

