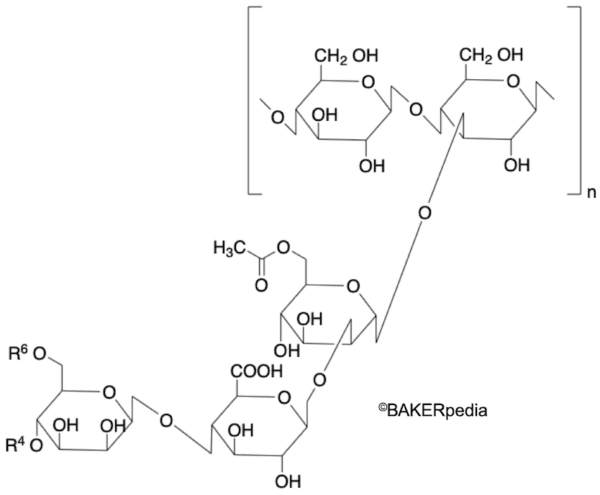
Chemical structure
Origin
Xanthan gum is by far the most widely used gum in the food industry. It is obtained from the fermentation of simple sugars by Xanthomonas campestris. This process was discovered in the 1950s, by scientists from the US Department of Agriculture. In 1960, the industrial production of xanthan gum began. By 1964, it became commercially available, and in 1969 the FDA approved its use as a food additive. Shortly after, it was approved in Europe in 1974.
Function
Xanthan gum has several functions in baked goods:
- Thickening agent: due to its ability to form highly viscous solutions even at low concentrations and in a wide temperature range (0-100°C / 32-212 °F).
- Stabilizing agent: provides oil-water emulsions and freeze-thaw stability by producing a web that avoids aggregations.
- Gelling agent: it can form gels when mixed with locust bean or tara gum.
- Dough improver: improves dough properties such as elasticity, and gas retention during proofing and baking.
- Texturizer in gluten-free baked goods.
- Shelf-life improver
Nutrition
Xanthan gum is not digested by the human body, and so it doesn’t provide any calories. Some health benefits associated with xanthan gum consumption include: reduction in blood sugar and cholesterol levels. It may aid in weight management.
Commercial production
Xanthan gum is produced commercially by the following process:
- Fermentation: glucose, sucrose or starch are placed in a batch reactor with Xanthomonas campestris culture and are allowed to ferment. It’s critical to maintain pH at or above 5. Optimum temperature for this step is 28 °C (82.5°F).
- Pasteurization: the fermented solution is pasteurized .
- Recovering: isopropyl alcohol is added to recover the polysaccharide, and precipitate it by solvent addition.
- Drying: precipitated xanthan gum is air dried, or spray dried to approximately 11% moisture.
- Milling: the obtained powder is milled to the desired particle size.
- Packing: the powder is packed and sealed for distribution.
Several grades of xanthan gum are commercially available. The most commonly used is the white fine powder.
Application
Xanthan gum is used in several baked goods, such as: cookies, cakes, bread, biscuits and muffins. It improves several quality parameters, mainly storage stability under freezing conditions. These attributes are due, in part, to xanthan’s ease of dissolution in hot and cold water, its compatibility with salts and resistance to enzymes present in food systems.
| Bakery system | Level | Effect |
| Wet batters | 0.05% |
|
| Pancake batter | 0.05% |
|
| Baked goods | 0.05% |
|
| Refrigerated dough | 0.05% |
|
| Pie or baked goods filling | – |
|
Regulations
Xanthan gum can be safely added to food products according to the FDA under conditions established by the CFR Title 21 Volume 3 Part 172.
In the European Union, xanthan gum (E 415) and its quality parameters are established by the EU Commission Regulation NO 231/2012.
References
- Damodaran, Sr, Parkin,K. L and Fennema,O.R. Fennema’s food chemistry. 4 th ed., CRC press, 2008.Blanshard, J.M.V, and Mitchell, J.R . Polysaccharides in food. Elsevier, 2013, pp. 138-141.
- Blanshard, J.M.V, and Mitchell, J.R. Polysaccharides in food. Elsevier, 2013 pp.263-282.
- Phillips, G. O., and Williams, P.A. Handbook of hydrocolloids. 2 nd ed., Boca Raton, FL: CRC press, 2009, pp. 186-202.
- Food and Drug Administration (FDA). US Department of Health and Human Services. CFR Code of Federal Regulations Title 21, Part 172 Food Additives Permitted For Direct Addition To Food For Human Consumption, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.695 . Accessed 26 August 2020.
- European Commission (EC). Commission Regulation NO 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council Text with EEA relevance. Official Journal of European Communities, 09 March 2012.

