Origin
Cellulases produced by symbiotic bacteria are found in ruminants and most recently in green algae.
Microbial cellulases can be obtained from fungal fermentations, usually using Aspergillus niger and Trichoderma sp.
Function
Cellulase helps minimize the negative effects of bran addition to bread formulas. Despite being a highly valued nutritional component, the bran imposes additional technological challenges to bakers since it highly disrupts foam structures and limits gluten network formation.
The following attributes can be obtained from using cellulase in bread:
- Increased pan flow through free-water release
- Superior gas retention for optimum oven spring and product volume
- Stronger and more continuous gluten network to minimize gas bubble coalescence
- Finer and more even gas cell structure in the crumb
- Strong synergy with glucose oxidase (in this case, cellulose provides glucose substrate to the oxidizing enzyme to enhance strengthening functionality)
- Softer crumb and longer shelf-life in variety bread
- Darker and more even crust color in low-sugar fiber breads
There are 4 major types of cellulase:
- Endo-glucanases
- Cellobiohydrolase
- Exo-glucohydrolase
- Β-glucosidase
The following table summarizes the key aspects of each type of cellulase:2
| EC number | Reaction catalyzed | Additional comments |
|---|---|---|
| 3.2.1.4 Endo-splitting enzyme Endo-glucanase | Random endo-hydrolysis of β-(1,4)-D- and β-(1,3)-D-glucosidic linkages in cellulose (glucan) polymeric chains.The products include glucose, cellobiose, and cellodextrins of various sizes. | Inactive against crystalline celluloses such as cotton.It can hydrolyze amorphous celluloses (including amorphous regions of crystalline celluloses) and soluble substrates such as thickeners carboxymethylcellulose (CMC) and hydroxymethylpropylcellulose (HPMC).
Endoglucanase activity results in a rapid decrease in viscosity and a steep increase in reducing groups which can then participate in Maillard reactions. Its action is pretty similar to fungal alpha-amylase. |
| 3.2.1.91 Exo-splitting enzyme | 1,4-β-D-glucan cellobiohydrolaseDegrades amorphous cellulose by consecutive removal of cellobiose from the non-reducing ends of the glucan substrate. | When pure it can degrade microcrystalline cellulose.The rate of increase in reducing groups in relation to decrease in viscosity is much higher than for the endoglucanases. Endoglucanases and cellobiohydrolases act synergistically in the hydrolysis of crystalline cellulose.
Its action is pretty similar to cereal beta-amylase. |
| EC 3.2.1.74 Exo-glucohydrolase | Hydrolyzes consecutively the removal of glucose units from the non-reducing end of cellodextrins. | The rate of hydrolysis decreases as the chain length of the glucan molecule decreases.Its action is pretty similar to amyloglucosidase (AMG). |
| 3.2.1.21β-glucosidase
β-D-glucoside glucohydrolase |
Cleaves cellobiose into 2 glucose units, and removes glucose from the non-reducing end of small cellodextrins. | Unlike the exo-glucohydrolase, the rate of β-glucosidase increases as the size of the glucan chain decreases, with cellobiose being hydrolyzed the fastest.Its action is pretty similar to the combination of maltase and AMG. |
Commercial production
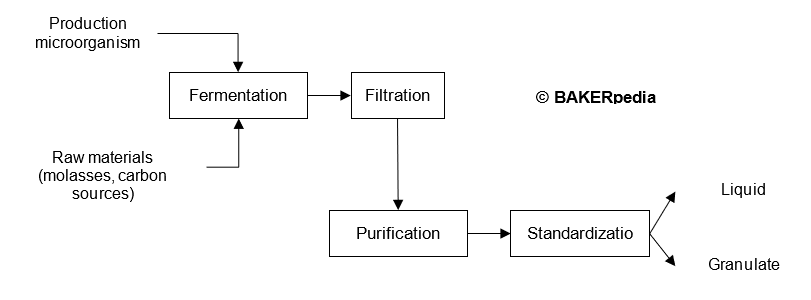
Large scale production of cellulase relies on fermentation and downstream processing using modern biotechnology.
The following block diagram summarizes the commercial production of cellulase:
Application
Fungal cellulase has a molecular weight of about 63,000 Da, a pH optimum of 5.5 to 6.0, and is stable for 5 minutes at 100°C at pH 7. The enzyme is inhibited by heavy metal ions, by sulfhydryl reagents, by oxidizing and reducing agents, and by glucose.2
Cellulase activity can be determined by measuring the rate of reducing groups released from cellulose.
Regulation
Similar to other enzymes, food cellulases are GRAS (Generally Recognized as Safe) and often considered a food processing aid in the US. The FDA regulates their origin (food-compatible) and establishes limits to their use (if applicable) based on GMP.
References
- Rosell, C.M., and Dura, A. “Enzymes in Bakeries.” Enzymes in Food and Beverage Processing, CRC Press, Taylor & Francis Group, LLC, 2016, pp. 171–204.
- Whitaker, J.R. “The Glycoside Hydrolases.” Principles of Enzymology for the Food Sciences, Marcel Dekker, Inc,, 1994, pp. 414–416.

