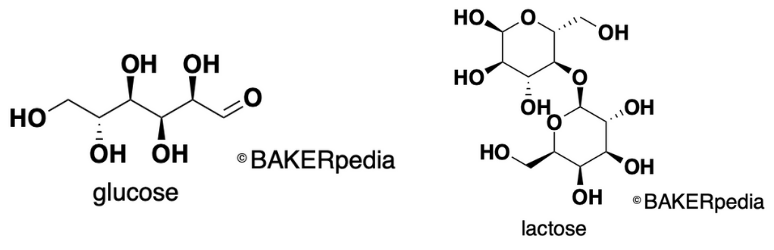
Structure of glucose and lactose
Function
All mono- and disaccharides have reducing capabilities with smaller sugars reacting faster than larger ones. Reducing sugars impart several effects in baked goods, such as:3
- Sweetening: Small reducing sugars are sweeter than larger ones
- Tenderizing: interfere with gluten formation, protein coagulation and starch gelatinization
- Shelf life improvement: limit the amount of water available for microbial deterioration
- Provide food for yeast: common sugars can be fermented by yeast
- Color and flavor: reducing sugars such as glucose upon heating in the presence of a free amino group undergo non-enzymatic browning reactions. Products of this reaction include desirable color compounds mainly melanoidins.2 Caramelization, on the other hand, takes place by heating sugar in acidic media to produce unique colors, flavors and aromas.2
Nutrition
Typical carbohydrates like glucose, fructose and sucrose have a caloric value of 4 kcal/g. One drawback of including reducing sugars in baking is the potential development of acrylamides derived from the second order reaction of reducing sugars and L-aspargine. Acrylamide is a known neurotoxicant and carcinogenic agent found in some baked, fried or roasted food (<1.5 ppm).2
Application
Reducing sugars have important contributions to baked goods such as breads, muffins, cookies, bagels, tortillas, cakes and pastries. While Maillard reactions can occur at room temperature, in the case of milk solids and lactose, caramelization requires high temperatures 160 -170 °C (320-340°F).3
Efficiency of reducing sugars in undergoing browning reactions follows this order:3
fructose > glucose > lactose > sucrose > maltose > isomalt
Some consideration when working with reducing sugars include:3
- Type of flavors developed: Maillard reactions produce flavors associated with roasted cocoa, roasted coffee and toffee. Caramelization provides a burnt sugar flavor.
- Dairy products are more susceptible to room temperature Maillard reaction and may produce off flavors.
- Temperature: the higher the temperature the more intense is the browning
- The presence of metals increases the rate of the browning reaction, some unrefined syrups can be found in malt, molasses, maple and honey.
- Acids and alkalis affect the rate of browning, a small amount of baking soda may increase the reaction rate. Meanwhile, cream of tartar or buttermilk reduces it.
- Water activity: ideal water activity is between 0.6-0.7
- Bakers must formulate recipes considering a content of 10-20% of reducing sugar per total sugar content. The concentration of liquid reducing sugar is around 70-80%.
FDA regulations
Glucose and fructose are generally recognized as safe by the FDA for its intended use.4,5
References
- Considine, John Anthony, and Elizabeth Frankish. A complete guide to quality in small-scale wine making. 1 st ed., Elsevier Academic Press, 2014, pp.155-187.
- Damodaran, Sr, Parkin,K. L and Fennema,O.R. Fennema’s food chemistry. 4 th ed., CRC press, 2008.
- Figoni, P. How Baking Works: Exploring The Fundamentals Of Baking Science. 2nd ed., John Wiley & Sons, Inc., 2008.
- Food and Drug Administration (FDA). US Department of Health and Human Services. CFR Code of Federal Regulations Title 21, Part 184 Direct Food Substances Affirmed As Generally Recognized As Safe, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1857&SearchTerm=corn%20sugar, Accessed 21 July 2020.
- Food and Drug Administration. “High Fructose Corn Syrup Questions and Answers”.Food Additives and Petitions, 04 Jan 2018. https://www.fda.gov/food/food-additives-petitions/high-fructose-corn-syrup-questions-and-answers. Accessed 21 July 2020.

