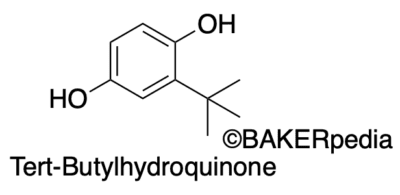
Chemical structure
Origin
TBHQ is obtained from a hydroquinone substituted with a tert-butyl group. In 1972, it was FDA-approved for its use as a food antioxidant.1,2,3
In 1998, TBHQ was authorized as a food additive by the FAO/WHO Expert Committee, and in 2004 its approval was confirmed by the European Food Safety Authority. 1,2,3
Function
In baking, TBHQ provides many benefits: 1,2
- Antioxidation: by preventing oxidation and rancidity of unsaturated oils and animal fats.
- Antibacterial: prevents the proliferation of certain bacteria.
- Shelf-life extension: via reducing fat oxidation.
Nutrition
TBHQ is an approved food additive. However, recent consumers’ push for replacing TBHQ and other synthetic antioxidants with natural products have convinced food manufacturers to seek other natural alternatives. TBHQ acceptable daily intake (ADI) should not exceed 0.2 mg/kg of body weight.1,2,3
Commercial production
TBHQ can be industrially produced through the following process:4
- Mixing: phosphoric acid is mixed with hydroquinone in a reactor.
- Heating: the solution is further heated to 55 – 65 oC (131 – 149 oF). t-butanol is then added and heated up to 75 – 85 oC (167 – 185 oF) for 2 -3 hours.
- Centrifugation, washing and dehydration
- Mixing: crude TBHQ is mixed with acid solution and heated up to 92 – 95 oC (197.6 – 203 oF).
- Second centrifugation: the TBHQ solution is centrifuged once the solution cools to 45 oC (113 oF).
- Second reaction: TBHQ is mixed with ethanol solution.
- Filtering: once completely dissolved, TBHQ is filtered and cooled naturally to allow for crystal formation.
Application
TBHQ is commonly incorporated into the lipid phase in baking formulations. It can be used in combination with other common antioxidants such as BHT and BHA but not with propyl gallate (PG).1,2
In lipid-containing food formulations, TBHQ is highly effective due to the following properties: 1,2
- High antioxidative impact in frying oils and shortenings.
- Similar to BHA and BHT in its oxidative ability..
- Soluble in fats.
- Does not react with copper and iron and does not produce complex compounds. .
- Does not discolor food products.
- Does not produce off flavors.
- Citric acid or monoglycerides citrates can be added to enhance its lipid stabilizing properties.
Usage level of TBHQ in the Europe and USA:1,3
| Usage level | USA | Europe |
|---|---|---|
| Fats and oils | 0.2% | 200 mg/l |
| Bakery products | 0.2% | 200 mg/l (only in cake mixes) |
| Cereal | 0.2% | 200 mg/l (only pre-cooked cereals) |
Regulations
The FDA considers TBHQ to be safe for its direct addition to food products when used in accordance to the following conditions:5
- TBHQ has a melting point of not less than 126.5 oC (259.7 oF).
- It contains 99% TBHQ.
- It can be used alone or in combination with BHT and/or BHA.
- TBHQ content can’t exceed 0.02% of the product fat or oil content, including volatile essential oil content.
In the EU, TBHQ (E319) is considered safe by the European Food Safety Authority, and has an acceptable daily intake of 0.7 mg/kg. The EU Commission Regulation No 1333/2008 regulates TBHQ use as a food additive.3
References
- Burdock, George A. Encyclopedia of food and color additives. Vol. 1. CRC press, 1997.
- Furia, Thomas E. CRC handbook of food additives. Vol. 1. CRC press, 1973.
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2016. Statement on the refined exposure assessment of tertiary-butyl hydroquinone (E 319). EFSA Journal 2016;14(1):4363, 26 pp.doi:10.2903/j.efsa.2016.4363
- 卢俊青. Tbhq Preparation Process. No. WO2015149586A1, 2014.
- Food and Drug Administration (FDA). US Department of Health and Human Services. CFR Code of Federal Regulations Title 21, Part 172 Food Additives Permitted For Direct Addition To Food For Human Consumption ,https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.185 , Accessed 26 November 2020.

