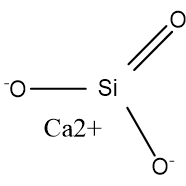
Function
Anticaking agents are substances added to food powders to prevent caking, lumping, or aggregation and to improve flowability. Anticaking agents function by different mechanisms in the presence of crystalline ingredients water absorption, water repellent and prevention of crystal-to-crystal contact.1
The mechanism of calcium silicate is by water absorption. It is reported to absorb water at 2.5 times its weight to pull moisture away from the host ingredient.1 Calcium silicate has high water affinity. It traps water and retains it internally. It can also retain moisture during desorption, the release of one substance from another.
Commercial production
Calcium silicate is produced from lime, hydrochloric acid, and sodium silicate. Burnt lime is treated with hydrochloric acid to produce calcium chloride. The calcium chloride solution is then treated with a clear sodium silicate once calcium silicate is precipitated out. The precipitate is centrifuged, washed, dried and packed in suitable containers.2
Application
Different anticaking agents function by different mechanisms. Other examples of anticaking agents are silicon dioxide and calcium stearate. Silicon dioxide functions by creating a barrier between host particles, which could decrease powder stickiness and possibly delay deliquescence, and by absorbing water. Calcium stearate functions by the ability to coat the host crystal particles, acting as a water repellant due to its hydrophobic nature, thereby preventing crystal-to-crystal contact and reducing total moisture sorption.
When applying an anticaking agent, remember that other factors, like storage relative humidity (RH) and storage time, have a big influence on caking.
Calcium silicate has other industrial uses besides anticaking agent. It is commonly used as a safe alternative to hazardous asbestos for high temperature insulation materials. It is also used as a sealant to cured concrete or the shells of fresh eggs. It reacts with calcium hydroxide or carbonate to form calcium silicate hydrate, sealing the pores with a relatively impermeable material.
FDA regulation
Calcium silicate, including synthetic calcium silicate, is generally recognized as safe when used as anticaking agent at levels not exceeding 5 percent in baking powder, two percent in other food, two percent in animal feed, by weight, regulated by FDA in the Code of Federal Regulations.3-4
References
- Lipasek, Rebecca A., Julieta C. Ortiz, Lynne S. Taylor, and Lisa J. Mauer. “Effects of Anticaking Agents and Storage Conditions on the Moisture Sorption, Caking, and Flowability of Deliquescent Ingredients.” Food Research International 45.1 (2012): 369-80.
- “CALCIUM SILICATE.” Process Flow Sheets. blogspot.com/2011/04/calcium-silicate.html Accessed 16 Jan. 2017.
- “21CFR182.2227.” Code of Federal Regulations. U.S. Food & Drug Administration, 1 Apr. 2016.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.2227&SearchTerm=calcium%2Bsilicate Accessed 11 Jan. 2017.
- “21CFR573.260.” Code of Federal Regulations. U.S. Food & Drug Administration, 1 Apr. 2016.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=573.260 Accessed 11 Jan. 2017.