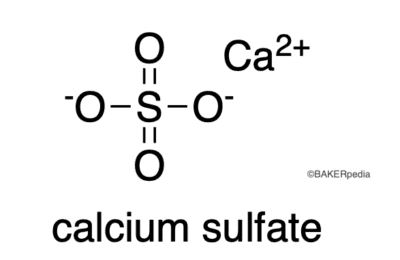
Chemical structure
Function
Calcium sulfate serves several functions in baked goods:1,2
- Anticaking agent: prevents powder caking, lumping or agglomeration.
- Coloring adjunct: aids in the preservation of color in coatings.
- Dough strengthener: modifies starch and gluten to provide a more stable dough.
- Firming agent: prevents the collapse during processing.
- Flour bleaching treatment
- Leavening aid: provides food for yeast improving leavening.
- Nutrient supplement: provides calcium for nutritional value.
- Stabilizer and thickener: provides body and improved consistency.
- Texturizer: improves baked good texture.
- pH regulator: works as a pH buffer and processing aid
Nutrition
Calcium sulfate can be used as a calcium supplement in the fortification of bread and flours. It is considered safe for daily consumption up to 8.5 g of anhydrous calcium sulfate per day, this value corresponds to 2500 mg of calcium per day.3
Commercial production
Calcium sulfate is commercially produced through two process, depending on the type:2
USG Terra Alba
- Grinding: high purity gypsum (with 20% water of crystallization) is ground to desired particle size.
- Air separation: gypsum is heated, at temperatures above 50oC (122oF,) part of the crystallization water is released. At 180 oC (356oF) over 75% of the water is released and complete evaporation is achieved when temperature exceeds 400 oC (752 oF).
Snow White Filler
- Calcing: gypsum is heated to temperatures from 300 – 450 oC (572 – 842 oF) in the presence of steam in a calcinating reactor.
- Grinding: anhydrous calcium sulphate is ground to desired particle size.
Application
Calcium sulfate is used mainly in the production of bread, and in the fortification of bread flour, cookies, brownies and breads. When using calcium sulfate some consideration must be taken into account:2,4
- As a flour bleaching agent: it is mixed with benzoyl peroxide at a ratio of no more than 6:1 by weight.
- As a nutritional supplement: for calcium enrichment 0.64% of calcium sulfate and 0.6% of an emulsifier by flour weight can be added for optimum enrichment.
- As a sodium sulfate substitute: it can replace up to 32% of sodium in the manufacturer of brown bread without affecting palatability and overall product quality.
- As a pH regulator: it can be used at a usage level of 0.1 – 0.6%.
Typical calcium sulfate usage levels in common food products:2
| Food product | Usage level |
|---|---|
| Baked goods | 1.3 % |
| Confections and Frostings | 3.0 % |
| Frozen dairy desserts | 0.5 % |
| Gelatins and Puddings | 0.4 % |
| Pasta and Grain products | 0.5 % |
| All other products | 0.07 % |
Regulations
Calcium sulfate is considered GRAS by the FDA for its direct addition to food products, in levels that do not exceed good manufacturing practices.2
In the EU, calcium sulfate ( E 516) is considered safe and it’s regulated by the EU Commission No 231/2012.5
References
- Lewis, Richard J. Food additives handbook. Springer Science & Business Media, 1989.
- U.S. Department of Health and Human Services.” Direct Food Substances Affirmed As Generally Recognized As Safe”.Title 21 Code of Federal Regulation, Part. 184. April 2019. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1230&SearchTerm=calcium%20sulfate . Accessed 17 October 2020.
- European Food Safety Authority (EFSA). “Calcium sulphate for use as a source of calcium in food supplements‐Scientific opinion of the Scientific Panel on Food Additives and Nutrient Sources added to food.” EFSA Journal 6.10 (2008): 814.
- AUSIMIN. “Calcium Sulphate for the Baking Industry”. Technical Specifications. AUSIMIN. Available at: http://ausimin.com.au/PDF/1.%20Ausimin%20Terra%20Alba%201-%20Calcium%20Sulphate%20for%20the%20Baking%20industry.pdf . Accessed 17 October 2020.
- European Commission (EC). Commission Regulation NO231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council . Official Journal of European Communities, 09 March 2012.

