How it works
Chemical leavening systems can be formulated and added individually to the recipe. Or, they can be added as a prepared blend, known as baking powder.
There are four primary considerations when formulating chemical leaveners:1
- Establish the how much bicarbonate (base) is required in the formula
- Determine the speed of the leavening system. Does gas need to be released quickly during mixing/bench time, or slowly until the product is in the oven?
- Select the type of leavening acid
- Establish the percent of bicarbonate to be neutralized with acid*
* Neutralizing value (NV): pounds of base neutralized by 100 pounds of acid. NV indicates the quantity of base to be neutralized in order to release all the available CO2. NV directly impacts the pH of the batter or dough.2
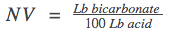
When amount of bicarbonate is known, then the amount of acid can be calculated:
Application
When choosing a leavening system, here are some things to consider:
- What is the product application?
- Inorganic or organic leavening acid?
- Clean label or regular product formulation?
- Regulations for the product application?
- Is chemical composition important (e.g. calcium, sodium, magnesium, aluminum)?
Bases
Bases available to the baker:1,2
| Bicarbonate Type | Lb CO2 Released / Lb Bicarbonate | Theoretical Substitution | Application |
| Sodium bicarbonate, or baking soda (NaHCO3) | 0.52 | 1.00 | All sweet goods |
| Potassium bicarbonate (KHCO3) | 0.44 | 1.19 | Low sodium cakes |
| Ammonium bicarbonate (NH4HCO3) | 0.56 | 0.94 | Very low moisture products like crackers and biscuits |
Bicarbonates produce carbon dioxide gas by neutralizing acids upon contact with water. Also, by thermal decomposition during heating.
Sodium bicarbonate levels in baked goods
| Product | % Baking Soda (based on total formula weight) |
| Batter-based (cakes) | 0.5–1.0 |
| Cookies | 0.4–0.6 |
| Muffins and corn bread | 1.2–2.0 |
| Cake doughnuts | 0.7–1.0 |
| Pancakes and waffles | 1.4–2.0 |
| Biscuits | 1.2–2.0 |
Acids
Acids available to the baker:1,2,3
| Baking Acid | Neutralizing Value (NV)* | Reaction Rate | Considerations / Application |
| Fumaric acid | 145 | Very fast |
|
| Tartaric | 116 | Very fast | |
| Cream of tartar | 45 | Fast | |
| Ascorbic | 52 | Very fast | |
| Propionic | 115 | Very fast | |
| Lactic | 93 | Very fast | |
| Citric | 159 | Very fast | |
| Monocalcium phosphate monohydrate (MCP) | 80 | Moderately fast | Pancake mixes, angel food cake, double-acting baking powder. |
| Monocalcium phosphate anhydrous (aMCP) | 83 | Delayed fast | Self-rising flour, pancake and waffle mixes. |
| Sodium acid pyrophosphate (SAPP) | 72 | Slow |
|
| Glucono Delta Lactone (GDL) | 45 | Delayed / slow | Canned dough, chemically-leavened pizza. |
| Sodium aluminum phosphate anhydrous (SALP) | 100 | Very slow |
|
| Sodium acid sulfate (SAS) | 104 | Very slow | Used in combination with fast-acting leavening acids. |
| Dimagnesium phosphate (DMP) | 40 | Heat activated | Cake mixes |
| Dicalcium phosphate dihydrate (DCPD) | 33 | Heat activated | Cake mixes |
*Based on sodium bicarbonate
The speed of the reaction, or how fast the leavening system produces gas in dough or batter, is affected by:1,2
- Acid type and its reaction rate is influenced by strength (% dissociation in aqueous medium), and acid solubility.
- Particle size of bicarbonate. The larger the granulation, the smaller the exposed surface area. Also, the longer it takes to fully solubilize and react.
- Use of encapsulated base or leavening acid(s).
- Temperature. The higher the temperature, the higher the solubility of acids in water. Also, the faster the reaction rate.
- Water activity. Excess water is needed as dispersing medium. Ingredients such as calcium, sugar, starch, gums, and hydration level affect the reaction rate.
Chemical leavening systems are the key for high-quality products. The volume, density, cell structure and texture of the baked goods are determined by the gas produced from the leavening system used in formula.
References
- Russell, E.B. Chemical Leavening Basics, AACC International, Inc., 2018, pp. 1–84.
- Heidolph, B.B. “Designing Chemical Leavening Systems.” Cereal Foods World, American Association of Cereal Chemists, W- 1996-0308-01 F., 1996, pp. 118–126.
- Brodie, J., and Godber, J. “Bakery Processes, Chemical Leavening Agents.” Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., 2007, pp. 171–195.

