Origin
EDTA was first discovered in 1935 by Ferdinand Munz. The most widely used synthesis is the alkaline cyanomethylation of ethylenediamine by means of sodium cyanide and formaldehyde.
The following reaction describes the synthesis of EDTA:
H2NCH2CH2NH2 + 4 CH2O + 4 NaCN + 4 H2O → (NaOOC CH2)2 NCH2CH2N (NaOOC CH2)2 + 4 NH3
Ethylenediamine + Formaldehyde + Sodium Cyanide + Water → tetrasodium EDTA + Ammonia
Another commercial method for producing EDTA is the two-step Singer synthesis. In this process, the cyanomethylation step follows a different path from the hydrolysis described above. Hydrogen cyanide and formaldehyde react with ethylenediamine to form insoluble EDTN (ethylenedinitrilo-tetraacetonitrile). The intermediate nitrile is treated with sodium hydroxide (a base) to form tetrasodium EDTA.3
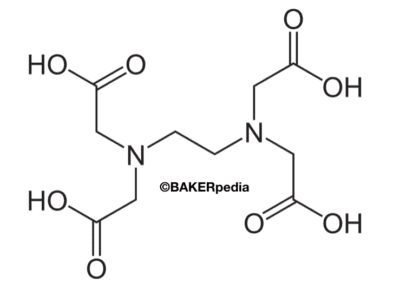
An Ethlenediaminetetraacetic acid (EDTA) molecule
Function
In the food and baking industry, EDTA has the following roles:
- Inhibits the enzymatic browning reactions in fruits and vegetables
- Inhibits oxidative rancidity in fat-based products (e.g., creams and spreads)
- Prevents decoloring of food pigments
- Inhibits the negative effects of certain metal compounds from process water (e.g., titanium chloride, potassium chromate, cuprous chloride, silver nitrate, mercuric chloride) on yeast cells (slowing down of dough fermentation)
Oxidation in food products is triggered/accelerated by:1
- High processing temperatures
- Presence of metals
- Exposure of food to light
- Enzymatic activity
- Fluctuations in temperature
Thanks to its multiple donor atoms, EDTA forms a very stable complex ion with various metals and free radicals so that they can be scavenged from the food products.2
Application
EDTA and its salt forms can be added to improve the shelf life, stability, and sensory characteristics of:
- Dressings
- Creams and frosting
- Margarine
- Natural colorings
- Fried bakery products
- Shelf-stable bakery products
EDTA is a very powerful chelation agent, with a recommended concentration of 100–300 parts per million. Its salts can be used as sequestrants in many foods, and even beverages. EDTA reacts negatively with ascorbic acid (vitamin C) and sodium bicarbonate. Therefore, it is not appropriate for use in chemically-leavened products like cake or donut batter. EDTA is usually used in shelf-stable filling or frosting.
FDA regulation
FDA 21 CFR Part 172 (Food Additives Permitted for Direct Addition to Food for Human Consumption) establishes the limits for the direct addition of EDTA to food products. The following are a few examples:
Type of EDTA Concentration in food products5,6
| EDTA (free-acid form) | According to good manufacturing practices |
| Calcium disodium EDTA (salt) | 25 ppm in fermented malt beverages
100 ppm in flavored spreads 200 ppm in eggs 75 ppm in margarine 100 ppm in frozen cut potatoes |
| Disodium EDTA (salt) | 75 ppm in dressings
150 ppm in aqueous multivitamin preparations 315 ppm in ready-to-eat cereal products containing dried fruit |
*1 ppm = 1 mg/Kg or L of product
References
- Belitz, H.-D. “Food Additives.” Food Chemistry, 4th ed., Springer-Verlag Berlin Heidelberg, 2009, pp. 455–456.
- Chang, R. “Transition Metals Chemistry and Coordination Compounds.” Chemistry, 10th ed., The McGraw-Hill Companies, Inc., 2010, p. 961.
- Hart, J.R. “Ethylenediaminetetraacetic Acid and Related Chelating Agents.” Ullmann’s Encyclopedia of Industrial Chemistry, John Wiley & Sons, Ltd., 2011, pp. 1–4.
- Msagati, T.A.M. “Minerals and Mineral Salts.” The Chemistry of Food Additives and Preservatives, John Wiley & Sons, Ltd., 2013, pp. 174–175.
- Moriartey, S. “Sequestrants.” Food Additives Data Book, 2nd ed., Blackwell Publishing Ltd., 2011, p. 868.
- U.S. Food and Drug Administration. “21 CFR 172 – Food Additives Permitted for Direct Addition to Food for Human Consumption.” 1 Apr. 2017, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172.

