High-speed bakers relied heavily on this oxidizing agent to troubleshoot plant issues like poor volume, product collapse, and excessive dough weakness. Its popularity soared in the ‘80s and ‘90s but dropped recently due to suspected carcinogenicity risks.
Function
Potassium bromate is used to improve the viscoelastic balance of dough, optimize its handling properties and build cohesiveness in the gluten matrix. These aspects are critical to improving gas retention, especially when the gluten film is at its weakest point due to maximum extension inside the proofer.1
KBrO3 accomplishes its function by promoting the oxidation of thiol or sulfhydryl groups (S-H) in proteins to disulphide bridges (S-S). This leads to cross‐linking of separate protein molecules and stronger and more continuous gluten aggregates that trap gases more effectively.1,2
The following illustration explains the mechanism of potassium bromate action:
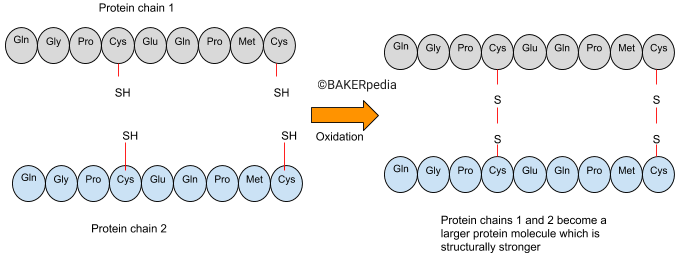
The increase in gluten size due to aggregation of proteins makes the dough more elastic, more resistant to deformation and less extensible.
Nutrition and health
There are health concerns associated with the use of potassium bromate, mainly its reported carcinogenicity in laboratory rats. As a consequence, it has been banned in many countries including all members of the European Union.3
Commercial production
Potassium bromate can be made by dissolving bromine in a hot potassium hydroxide solution, according to the following reaction:
6KOH + 3Br2 → 5KBr + KBrO3 + 3H2O
Application
Potassium bromate is a slow-acting oxidizing agent used in straight or no-time dough systems. These rely on mechanical dough development. Optimal conditions for potassium bromate are warm temperatures and a slightly acidic environment (pH less than 5.5). These conditions prevail during final proofing and initial stages of baking.
Action and typical usage of potassium bromate in bread formulations
| Oxidizing agent | Action | Acts mainly during | Typical usage (based on flour weight) |
| Potassium bromate | Slow- or late-acting | Proofing and early baking | 10–30 ppm |
Effects of potassium bromate on dough and finished product
| Over-scaling (over-oxidation) | Under-scaling (under-oxidation) | ||
| Dough |
|
Dough |
|
| Bread |
|
Bread |
|
Regulation
Because of health-related concerns, the use of potassium bromate is now illegal in many countries. Its use in the United States has been limited to 75 mg / Kg of flour.4 During thermal processing bromate is reduced to bromide, a harmless form of the oxidizer. However, the baking industry needs to take all necessary actions to reduce any possible bromate residues in the baked goods to safe levels, which a risk analysis conducted by the FDA has established at 20 ppb.3
Potassium bromate continues to be under review by the U.S. Food and Drug Administration (FDA).
References
- Rosentrater, K.A. “Flour Treatments, Applications, Quality, Storage and Transport.” Kent’s Technology of Cereals. An Introduction for Students of Food Science and Agriculture, 5th Edition, Woodhead Publishing, Elsevier Ltd., 2018, pp. 515–562.
- Sahi, S.S. “Ascorbic Acid and Redox Agents in Bakery Systems.” Bakery Products Science and Technology, 2nd edition, John Wiley & Sons Ltd, 2014, pp. 183–196.
- American Bakers Association (ABA) and AIB International. “Commercial Baking Industry Guide For the Safe Use of Potassium Bromate.” 2008.
- U.S. Food and Drug Administration. CFR – Code of Federal Regulations Title 21 Part 136 Bakery Products, April 1, 2018, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=136&showFR=1. Accessed 1 July 2019.

