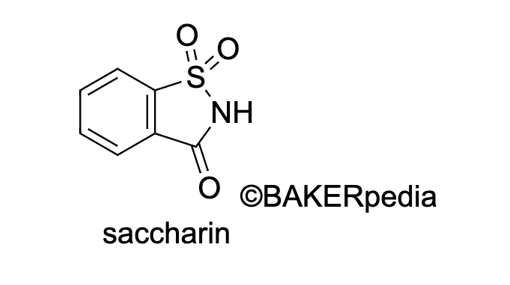
Chemical formula
Origin
Saccharin or o-sulfabenzamide was discovered in 1878 by Ira Remsen and Constantine Fahlberg of John Hopkins University in Baltimore. It was first manufactured in a pilot plant in New York by Fahlberg. During World War I, sugar rationing increased the consumption of saccharin and by 1917 this product became a household item.1
Function
Saccharin’s function in baked goods is to replace sucrose with a healthy sweetening alternative. This replacement may compromise some of the functional benefits of sucrose, mainly as a bulking agent.
Nutrition
Commercial saccharin nutritional value per 100 g:3
| Component | Grams |
|---|---|
| Carbohydrate | 89.11 |
| Water | 8.75 |
| Protein | 0.94 |
| Fat | 0 |
*Composition may not add to a 100 g
Saccharin is considered a no calorie sweetener due to the fact that it cannot be metabolized by humans.1 The Acceptable Daily Intake (ADI) of saccharin is 5 mg/kg.
Commercial production
Saccharin can be commercially produced using various processes with Ramsen and Fahlberg as the most commonly used :1
- Synthesis: toluene is treated with chlorosulfonic acid to produce ortho and para-toluene sulfonyl chloride.
- Ammonia treatment: ortho and para-toluene sulfonyl chloride are treated with ammonia to form ortho and para-toluenesulfonamide.
- Separation: ortho-toluenesulfonamide is separated from para-toluenesulfonamide.
- Oxidation: ortho-toluenesulfonamide is oxidized to obtain saccharin acid.
- Dissolution: saccharin acid is treated with sodium hydroxide to obtain sodium saccharine salt.
- Filtration
- Concentration
- Crystallization
- Separation from water
- Drying: to obtain a white powder.
- Sieving: to the appropriate granulometry.
Application
Saccharin is commonly used in the manufacture of low calorie food products such as:1
- Carbonated beverages
- Dressings and fillings
- Baked goods
- Chewing gums
- Sauces
Saccharin is stable under baking pH, moisture and temperature conditions.1
Characterized by its synergic effect with other sweeteners like aspartame and cyclamate, and bulking agents saccharin is commonly used in combination with other sweeteners to mask its bitter aftertaste.1
Considering the low concentration of saccharin required for baking, a bulking agent is often required to compensate for sucrose elimination from the formula.2
Regulations
Saccharin is considered safe for use in food products when following the conditions established by the FDA.4
In the EU, saccharin (E 954) was allowed for “fine bakery products for particular nutritional uses (diabetic food products)”. Since 2018, this category of “diabteic food products” has been eliminated , and thus fine bakery products are regulated by the Nutritional Health Claims Regulations 1924/2006 that doesn’t include the use of saccharin in bakery products.5
References
- O’Brien-Nabors, L. Alternative Sweeteners. United States: CRC Press, 2016.
- Hui, Y., Corke, H., De Leyn, I., Nip, W.N and Cross, N .Bakery products: science and technology. John Wiley & Sons, 2008.
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central, 30 October 2020. https://fdc.nal.usda.gov/fdc-app.html#/food-details/1103944/nutrients . Accessed 10 July 2021.
- Food and Drug Administration (FDA). US Department of Health and Human Services. CFR Code of Federal Regulations Title 21, Part 180 Food Additives Permitted In Food Or In Contact With Food On An Interim Basis Pending Additional Study, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=180.37&SearchTerm=saccharin , Accessed 10 July 2021.
- “EU Bans Use Of Artificial Sweeteners In Dietetic Bakery Products”. Apps.Fas.Usda.Gov, 2018, https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=EU%20Bans%20Use%20of%20Artificial%20Sweeteners%20in%20Dietetic%20Bakery%20Products_Brussels%20USEU_EU-28_2-5-2018.pdf . Bans Use of Artificial Sweeteners in Dietetic Bakery Products_Brussels USEU_EU-28_2-5-2018.pdf. Accessed 10 July 2021.

