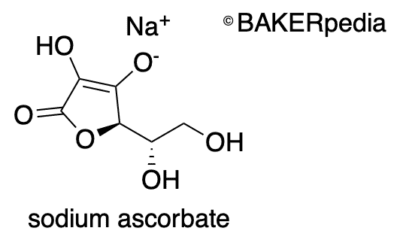
Chemical structure
Origin
Sodium ascorbate is found in citrus fruits and vegetables. It is synthesized from ascorbic acid and sodium bicarbonate. In 1935, the inhibiting effect of ascorbates and other salts on proteases was reflected in improved flour quality.
Function
This ingredient serves several purposes in baked goods:2,3,4
- Antioxidant and reducing agent: by reacting with free radicals it produces inactive compounds that disrupt free radical chain reactions.
- Dough conditioner: stabilizes the gluten network by reducing extensibility and increasing elasticity.
- Acidity regulator: contributes to acidity regulation unlike ascorbic acid that tends to increase dough acidity.
- Bread improver: helps improve volume, shape and texture of baked goods.
- Taste: provides a desirable tart flavor in candies and fruit juices.
Nutrition
Vitamin C is essential for human health mainly to prevent common cold, infections and gum disease and in maintaining proper immune function. One concern with this ingredient is its sodium content which may have undesirable consequences such as water retention, hypertension, kidney and heart disease.2,5
Commercial production
Sodium ascorbate can be industrially produced by two methods: reacting ascorbic acid with sodium bicarbonate, and via gulonic acid.
Ascorbic acid and sodium bicarbonate method:6
- Reaction: ascorbic acid is dissolved in water followed by addition of alkaline sodium bicarbonate.
- Solvent addition: to precipitate sodium ascorbate.
- Filtration: resulting sodium ascorbate is filtered and washed with a small amount of solvent.
- Drying: the obtained salt is air-dried.
Application
In addition to its dough conditioning power, it can help increase shelf life and dough quality of breads and rolls. It is best used in no-time doughs.
It can be added dry to the flour or as a solution in the dough, at a 0.1%. Substituting ascorbic acid by sodium ascorbate should take into consideration that one part of the salt is equivalent to 1.09 parts of ascorbic acid.7 Therefore, for 1-20 g ascorbic acid/Kg flour is equivalent to 0.9 – 18.35 g/100 Kg sodium ascorbate.2
The following table serves as a guide to the correlation between usage level, dough mixing and expected bread volume.2
| Compound | Level (ppm) | Optimum mixing speed (rpm) | Bread volume (cc) |
| Sodium Ascorbate | 0 | 240 | 3.840 |
| 36.70 | 208 | 3.855 | |
| 73.39 | 201 | 3.825 |
FDA regulations
Sodium ascorbate is generally recognized as safe by the FDA when used for its intended purpose, and following good manufacture practices.8
References
- “Sodium Ascorbate.” National Center for Biotechnology Information. PubChem Compound Database. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_ascorbate#section=Top. Accessed 22 July 2020.
- Mrak, E. M., and Stewart,G .F . “Advances in Food Research. Vol. I.” Advances in Food Research. Vol. V. 1958.
- Hui, Y. H . Handbook of food science, technology, and engineering. Vol. 4. 1 st., CRC press, 2005.
- Gioia, Luis Carlos, José Ricardo Ganancio, and Caroline Joy Steel. “Food additives and processing aids used in breadmaking.” Food Additives. IntechOpen, 2017.
- Kilcast, D and Angus,F. Reducing salt in foods: Practical strategies. 1 st ed., Elsevier, 2007.
- Holland, W. A. “Method of making sodium ascorbate.” U.S. Patent No. 2,442,005. 25 May 1948.
- Igoe, R. S. Dictionary of food ingredients. 5 th ed., Springer Science & Business Media, 2011.
- Food and Drug Administration (FDA). US Department of Health and Human Services. CFR Code of Federal Regulations Title 21, Part 182 Substances Generally Recognized As Safe, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1857&SearchTerm=corn%20sugar, Accessed 21 July 2020.