While sorbitol was first isolated from berries by French chemists in 1872, it was not commercially successful until saccharin established consumer demand for artificial sweeteners. By the mid 1950s, sorbitol was being industrially manufactured and was approved for use by the Federal Drug Administration (FDA) in 1971.
Composition
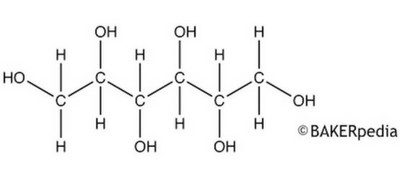
The chemical structure of sorbitol.
Sorbitol appears as a white hygroscopic powder, as a crystalline powder or as flakes or granules. It is highly soluble in water and slightly soluble in ethanol. The standard preparation is to dissolve a weighed quantity of it in water to obtain a solution with a concentration of about 10.0 mg of per ml. 3
Commercial production
Sorbitol has two-thirds the calories of sugar and, as mentioned earlier, is less sweet. It is poorly absorbed by the body, so it does not raise insulin levels as much as sugar does; nor does it promote tooth decay.
Sorbitol is a glucose molecule with two hydrogens added. The two extra hydrogens are on either side of what used to be the double bond connecting the oxygen to the carbon, which is now a single bond.
It is industrially manufactured by the catalytic hydrogenation of the glucose produced from cornstarch. 4 In addition to its use as an artificial sweetener, sorbitol is also used as a humectant in cosmetics and toiletries and as a liquid vehicle, stabilizer and sweetener in pharmaceuticals. 5
Application
Sorbitol frequently comes in powder form and is used as a sweetener or moisture-stabilizing agent in the production of confectionery, baked goods and chocolate, among many other products. Its moisture-stabilizing action makes it a good choice for products that tend to become dry or harden, and it helps maintain freshness during storage. 6 Sorbitol can withstand high temperatures and it combines well with other food ingredients such as gelling agents, fats and sugars.
FDA regulation
Sorbitol is certified as GRAS (generally recognized as safe) by the FDA. There are limits on the amount that can be used in food, and food containing an excess of 50 grams of sorbitol must be labeled with the statement: “Excess consumption may have a laxative effect.” For more information on the FDA guidelines for sorbitol use, please visit this FDA web page.
References
- “What Are Sugar Alcohols and How Do They Work?”Sugar.org. The Sugar Association, n.d. Web. Accessed on 06 June 2016.
2. Hicks, Jesse. “The Pursuit of Sweet: A History of Saccharin” | Chemical Heritage Foundation.”Chemheritage.org. Chemical Heritage Foundation, n.d. Web. Accessed on 06 June 2016. - “Sorbitol.”Joint FAO/WHO Expert Committee on Food Additives (JECFA)52.4 (1996): n. pg. Web. Accessed on 20 June 2016.
4. Silveira, M., and R. Jonas. “The biotechnological production of sorbitol.” Applied Microbiology and Biotechnology 59.4 (2002): 400-408.
5. “HSDB: D-SORBITOL.”Toxicology Data Network. U.S. National Library of Medicine, n.d. Web. Accessed 06 June 2016. - “Sorbitol.”Polyols Information Source. Calorie Control Council, n.d. Web. Accessed 06 June 2016.

