Origin
Tartaric acid is a natural acid which is widely distributed in nature in the form of 2 stereoisomers. It was first isolated by the alchemist Jabir Ibn Hayyan in Iraq in the 7th century. Resolving tartaric acid stereoisomers was done much later by Louis Pasteur in the 1840s.
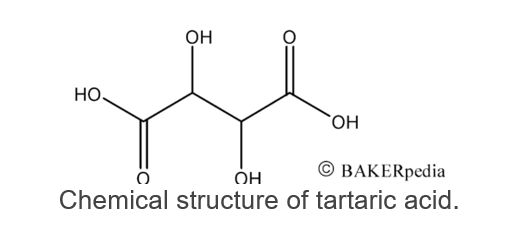
Similar to other organic compounds such as citric, acetic and lactic acids, tartaric acid has one unreacted carboxyl group (COOH).
Function
- Flavor enhancer (particularly in lime and grape-flavored beverages)
- Chemical leavener
- Anti-caking agent
- Firming agent
- Humectant
- Acidulant (helps reduce the pH of wine or juice, but not below pH 3.0)
- Antimicrobial or pH control agent, although to a lower extent than citric and other food acids
- Formulation and processing aid
Commercial production
Tartaric acid can be produced by natural and synthetic routes. The natural route involves the recovery of the reddish precipitated salt, potassium bitartrate, from argol, the sediment in wine vats. The synthetic chemical route involves the production of the racemic mixture of tartaric acid from maleic anhydride.
Commercially, tartaric acid is available as a colorless to white translucent fine crystals or granular crystalline powder. Due to its very high melting point (170°C), tartaric acid is a solid material under room conditions of temperature and pressure.1
Application
Characteristics of tartaric acid include:1
- Neutralizing value*: 116
- pK1: 2.98 (cannot reduce pH below this point)
- pK2: 4.34
- Has a sharp or bitter tart taste and is the most soluble of all acidulants
- When blended with citric acid, tartaric acid contributes tartness to sour apple and wild cherry flavor
- Solubility in water at 25°C: 156%
- Density at 20°C: 1.76 Kg/m3
*weight of baking soda required to neutralize 100 parts of leavening acid, i.e. indicator or acidity strength.
One remarkable application of tartaric acid is its key participation in the production of DATEM (diacetyl tartaric acid esters of monoglycerides).
Regulation
Tartaric acid is GRAS for miscellaneous and general-purpose usage in the USA with no limitation other than good manufacturing practices.2
Potassium acid tartrate (cream of tartar) is GRAS for miscellaneous and general-purpose usage in the USA with no limitation other than good manufacturing practices.2
Potassium acid tartrate use is limited in wine at 25 lb / 1000 gallons (50 mg / kg). Potassium bitartrate can be used to stabilise grape wine at a level not to exceed 35 lb / 1000 gallons (ca. 70 mg / kg) of grape wine.2
In the EU, L(+)-tartaric acid (E334) is a food additive. The Scientific Committee for Food established an acceptable daily intake of 30 mg/Kg body weight/day. 3
References
- Doores, S. “Acidulants.” Food Additives Data Book, 2nd edition, Blackwell Publishing Ltd., 2011, pp. 2–58.
- CFR – Code of Federal Regulations Title 21CFR184.1099. Accessdata.fda.gov. 1 Apr. 2016. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1099 Accessed 14 June 2021.
- Re-evaluation of L(+)-tartaric acid (E 334), sodium tartrates(E 335), potassium tartrates (E 336), potassium sodium tartrate (E 337) and calcium tartrate (E 354) as food additives .https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2020.6030.

