Origin
Malic acid is an alpha hydroxy organic acid which is widely found in fruits, such as apples, cherries, plums, and vegetables. This acid is sometimes referred to as a fruit acid or, more specifically, an apple acid.
As part of metabolic pathways, it is naturally found in every living cell.
Function
Functions of malic acid include:
- Antioxidant
- pH control agent
- Acidulant
- Preservative
- Flavor enhancer
- Flavor modifier
On the preservation function side of things, this acid is a powerful inhibitor of the growth of yeasts and some bacteria. It is more effective than acetic acid and lactic acid in inhibiting thermophilic bacteria, but is not as effective as lactic acid in suppressing the growth of Listeria monocytogenes.
Commercial production
It is commercially produced via large-scale fermentation and downstream processing.
Rhizopus oryzae and Aspergillus niger are almost always the preferred microorganisms used for organic acid production. These fungi are capable of producing different types of organic acids as primary metabolites.
Various residues from agriculture and industry can be used by different microorganisms as fermentable carbon sources. Such residues include cassava bagasse, coffee husk and pulp, apple pomace, and soybean and potato residues.
Application
How does malic acid compare to other food acidulants? Here is the relative sourness, in arbitrary units, of malic and other organic acids compared to citric acid:
- Citric acid: 100
- Fumaric acid: 55
- Tartaric acid: 70
- Malic acid: 75
- Succinic acid: 87
- Lactic acid: 107
- Glucono-delta-lactone: 310
In terms of tartness, 0.362 – 0.408 Kg of this acid is equivalent to 0.453 Kg of citric acid and to 0.272 – 0.317 Kg of fumaric acid. Similar concentrations of organic food acids may have different pH’s which typically range from 2-3 at 1% concentration.
Characteristics and properties of malic acid1
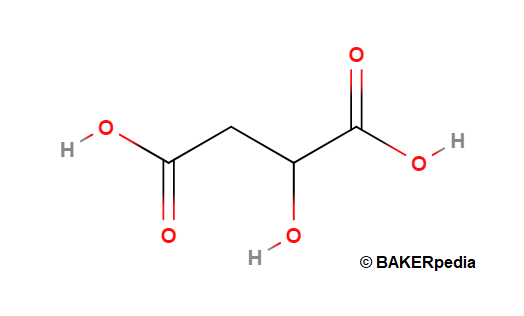
- Formula: C4H6O5
- Molecular weight (Da): 134.09
- Appearance: white crystalline powder or granule
- pK1: 3.46
- pK2: 5.21
- Melting point: > 100°C
- Flavor profile: smooth lingering taste (may help mask the bitter aftertaste of synthetic sweeteners, such as aspartame)
- Solubility (130 g / 100 mL distilled water at 20°C), which is slightly less soluble than citric acid.
A major drawback for using organic acids is their high cost. The most expensive organic acids include malic acid, citric acid, and tartaric acid (the most expensive of the commonly used food acids in the food and beverage industry).
Regulation
Malic acid and its salts have been confirmed by the U.S. FDA as GRAS. It can be used at levels not to exceed good manufacturing practice as a flavor enhancer and acidulant as well as a pH control agent at levels varying from 6.9% for hard candy to 0.7% for miscellaneous uses.2
Similarly, In the EU, malic acid (E296) is allowed as a food additive with no limit on daily intake when used following good manufacturing practice. 3
References
- Doores, S. “Acidulants.” Food Additives Data Book, 2nd edition, Blackwell Publishing Ltd., 2011, pp. 32–34.
- CFR – Code of Federal Regulations Title 21, Title 21 Chapter I Subchapter B, PART 184 DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1069, Accessed 5 december 2021.
- https://op.europa.eu/en/publication-detail/-/publication/28cb4a37-b40e-11e3-86f9-01aa75ed71a1/language-en. Accessed 5 December 2021.

