Origin
Surfactants can be either produced from chemical synthesis or extracted from biomaterials such as lecithin from sunflower seeds and eggs.
Function
Surfactants are amphiphilic compounds which have polar (hydrophilic) and non-polar (hydrophobic) moieties (regions) within the same molecule. The hydrophilic head associates with the aqueous phase and the hydrophobic tail associates with the lipid or air phase.
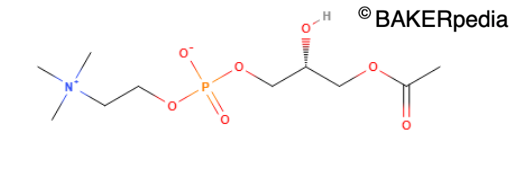
Lysophosphatidylcholine, anaturally occurring surfactant from lecithin
Surfactants work by reducing the interfacial tension between gases, solids and liquids in colloidal systems. Colloidal systems are composed of a dispersed and a continuous phase.
Example of colloidal systems
- Gases in liquids (foams)
- Cakes batter: Air / CO2 gas incorporated and dispersed in a continuous oil-in-water emulsion
- Beer
- Bakery foams for sponge cakes: Air/gas trapped and dispersed in a continuous phase of unfolded and cross-linked egg white proteins
- Gases in solids (solid foams)
- Bread dough: Air / CO2 gas incorporated and dispersed in a continuous solid yet highly hydrated gluten-starch matrix
- Liquids in liquids (emulsions)
- Mayonnaise
- Milk: Fat globules dispersed in a continuous water phase
- Liquids in solids (solid emulsions)
Application
Benefits from using surfactants in baked goods:
- Increase dough strength and improve dough handling during proofing and baking
- Increase tolerance to mechanical shocks that cause dough collapse
- Improve crumb structure by promoting an even and finer gas bubble structure
- Improve bread loaf volume by preserving colloidal stability
- Extend bread shelf-life by delaying starch retrogradation
- Improve product quality in gluten-free and high-fiber baked goods
Surfactants have different hydrophilic heads and hydrophobic tails. They are commonly classified by the ratio of hydrophilic-to-lipophilic groups (hydrophilic-lipophilic balance, or HLB).
HLB values determine whether hydrophilic (i.e., polar moieties) or lipophilic groups (i.e. non-polar moieties) are dominant. The HLB has values ranging from 0 to 20, indicating the emulsifier’s affinity to oil or water. If an emulsifier exhibits a low HLB value, this indicates that it is strongly lipophilic (water insoluble), exhibiting anti-foaming functionality. High HLB values imply the emulsifiers are hydrophilic (water soluble), exhibiting stabilizing properties for O/W emulsions.
In baking, high HLB values correspond to excellent dough strengthening functionality. On the other hand, Low HLB values highly correlate to poor dough strengthening effect. High amylose complexing index corresponds to excellent crumb softening functionality (anti-staling effect).
Key parameters of selected surfactants and their functionality1
| Emulsifier | HLB alue (Hydrophilic-to-lipophilic balance) | Amylose complexing index (ACI) |
|---|---|---|
| Sodium stearoyl lactylate / SSL / E481 | 10 – 13 (medium/high polarity) | 72 (very good) |
| DATEM / E472e | 9 – 10 (medium polarity) | 49 (fair) |
| Ethoxylated mono- and diglycerides of fatty acids | 10 – 12 (medium polarity) | Poor |
| Lecithin |
|
Poor |
| Glyceryl monostearate / E471 / GMS | 3 – 4 (low polarity) |
|
| Polyglycerol esters of fatty acids / PGE / E475 | 5 – 13 (medium polarity) | 30 – 34 (poor) |
| Polysorbates / PEG20 sorbitan esters / Tweens | 10 – 16 (high polarity) | 28 – 32 (poor) |
| Propylene glycol esters of fatty acids / PGMS / E477 | 3 – 4 (low polarity) | < 20 (poor) |
Regulation
In the US, surfactants used in the baking industry are considered GRAS by the FDA.
In the EU, surfactants with E-numbers are approved for food applications. 2
References
- Poirier, C.A. “Emulsifiers.” Food Additives Data Book, 2nd edition, Blackwell Publishing Ltd., 2011, pp. 318–363.
- Annex II of Regulation (EC) No 1333/2008. EU Food Additives Database. ec.europa.eu/food/food/fAEF/additives.

